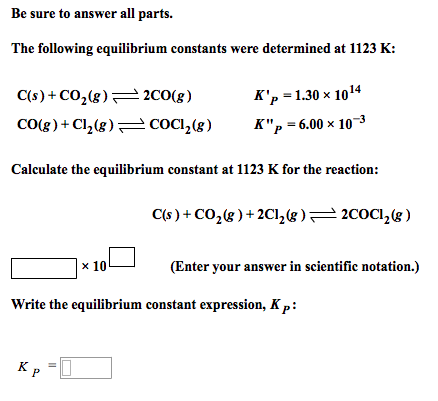
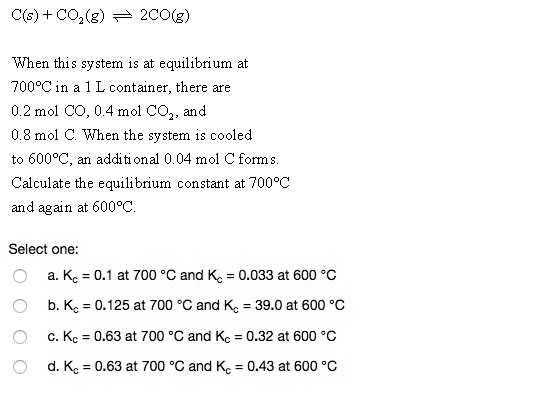
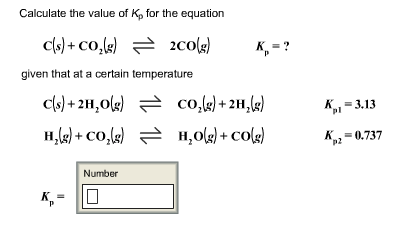
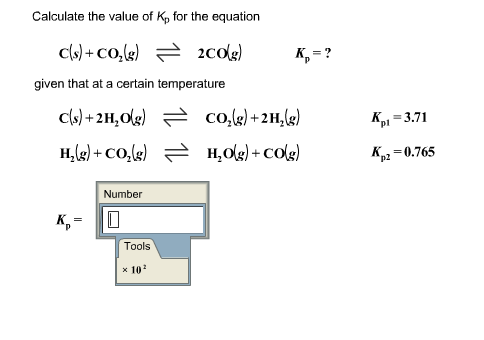
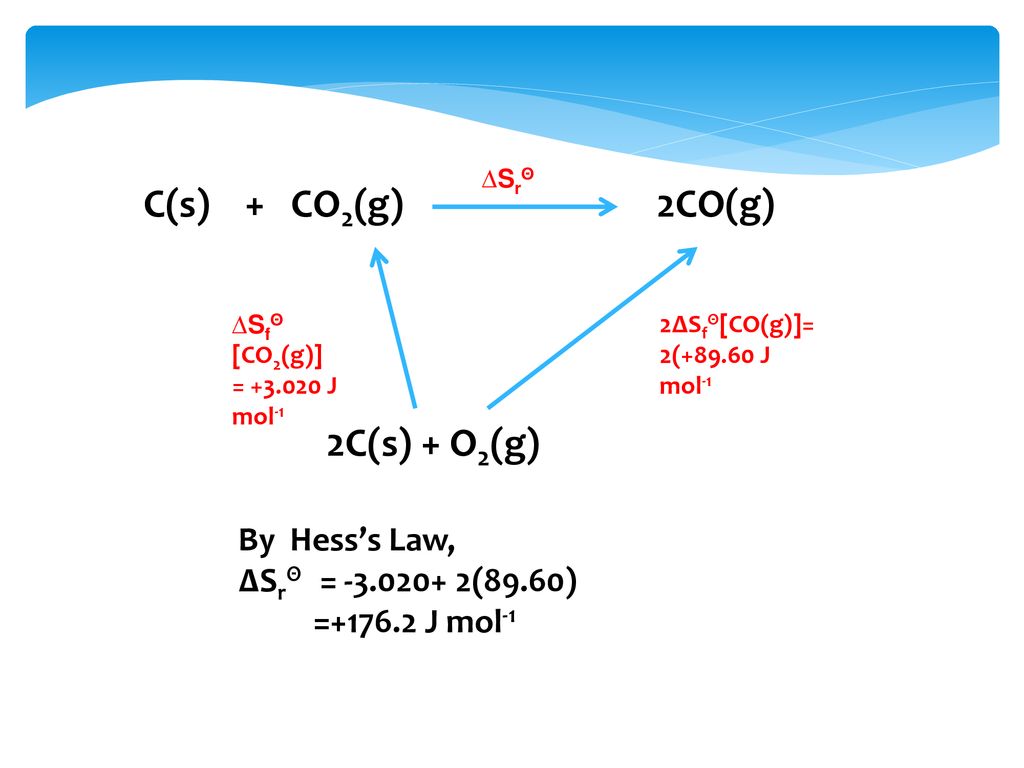
For the reaction: C (s)+ CO2 (g) 2CO (g) the partial pressure of CO2 and CO are 2 and 4 atm respectively at equilibrium. Then equilibrium costant for the reaction is -

For the reaction, c(s)+co2(g)⇌2co(g), the partial pressures of co2 and co are 2.0 and 4.0 atm respectively - Brainly.in

For the reaction, C (s) + CO2 (g) 2CO (g) , the partial pressures of CO2 and CO are 2.0 and 4.0 atm respectively at equilibrium. The Kp for the reaction is:

In the reaction C(s) + CO2(g) 2CO(g) the equilibrium pressure is 12 atm. If 50% of CO2 reacts calculate Kp .

Equilibrium concentrations of reactants and products in the reaction C... | Download Scientific Diagram

Values of the kinetic constant of the reaction C+CO2→2CO as a function... | Download Scientific Diagram
Given that C(g) + O2(g) → CO2(g) ∆H° = a kJ; 2CO(g) + O2(g) → 2CO2(g) ∆H° = -b kJ; Calculate the ∆H° for the reaction - Sarthaks eConnect | Largest Online

Solve this: â ‹Q3 For the equilibrium C(s) + CO2(g) ⇌2CO(g) KP = 63 atm at 1000 K - Chemistry - Chemistry in Everyday Life - 11997217 | Meritnation.com
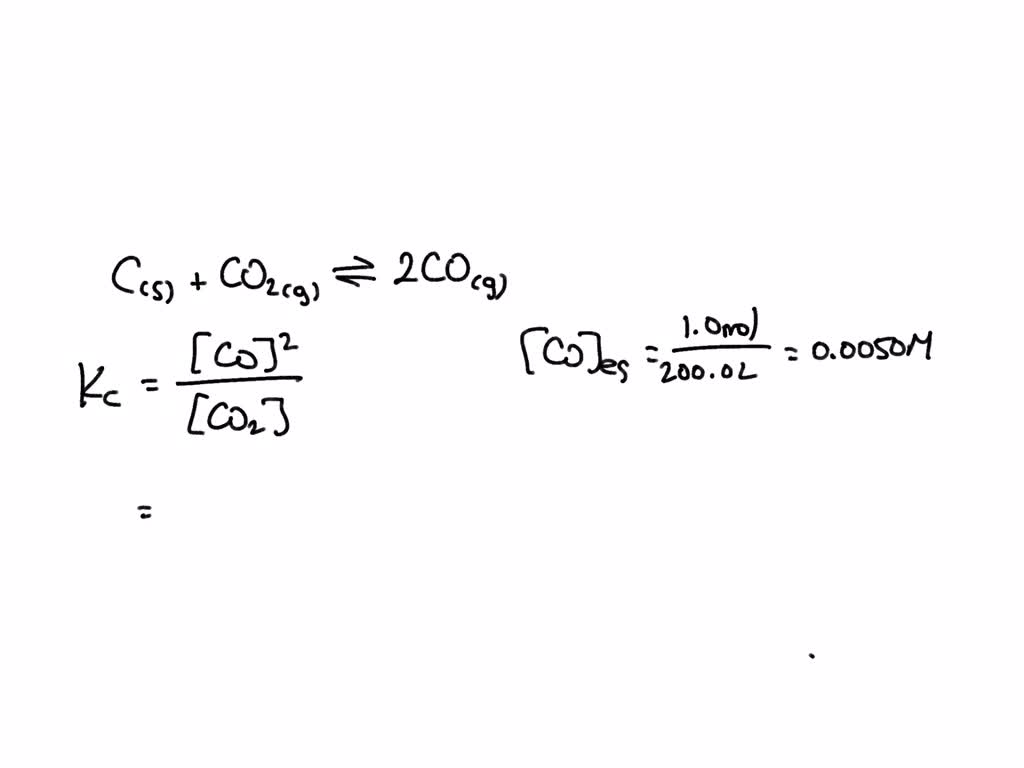
SOLVED: The reaction C(s) + CO2(g) ⇔ 2CO(g) occurs at high temperatures. At 700°C, a 200.0 L tank contains 1.0 mol CO, 0.20 mol CO2, and 0.40 mol of C at equilibrium.